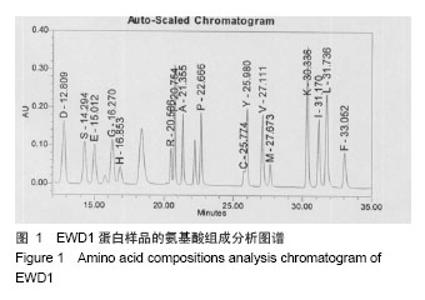
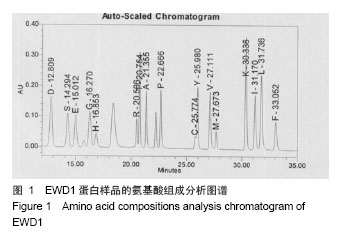
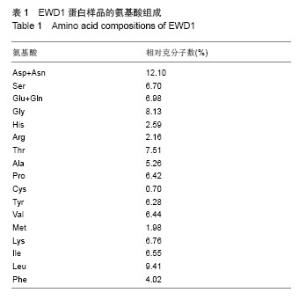
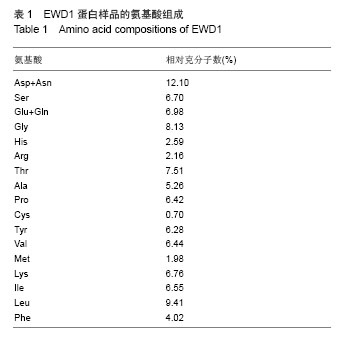
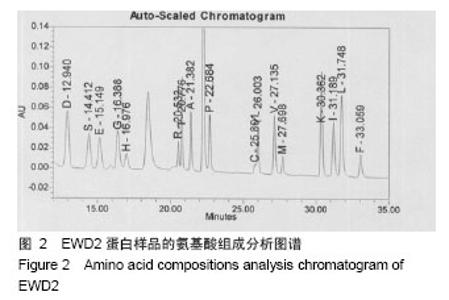
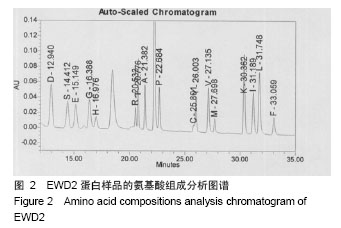
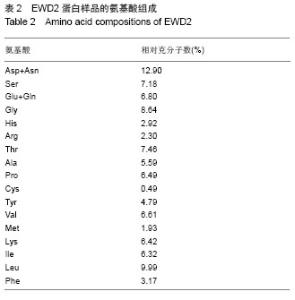
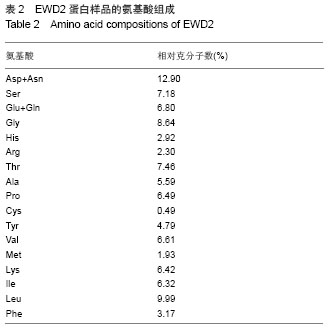
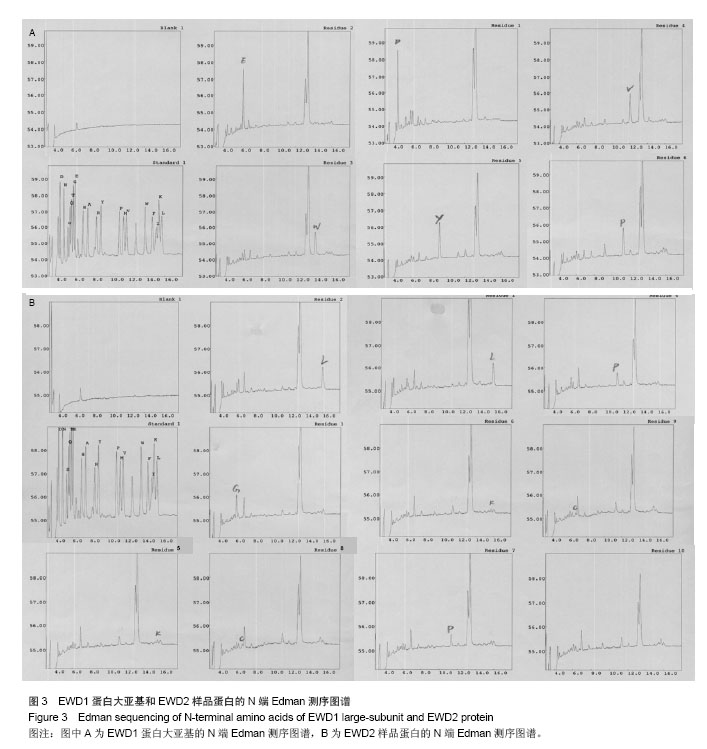
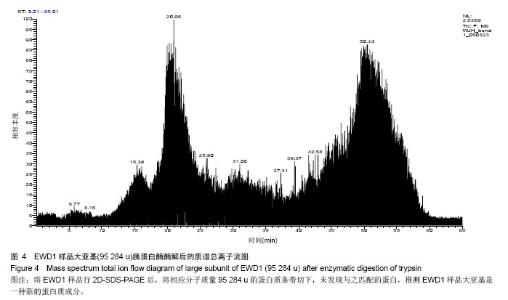
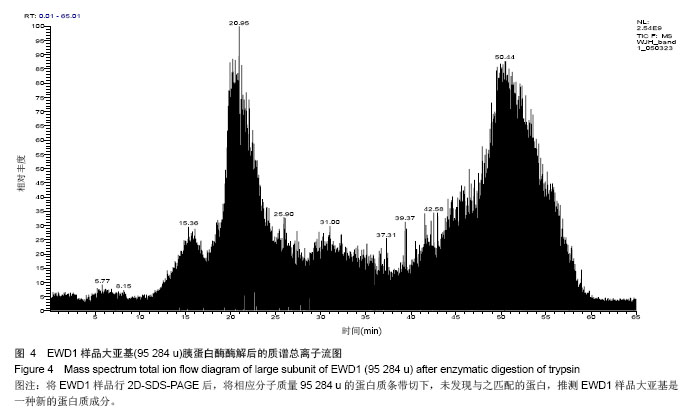
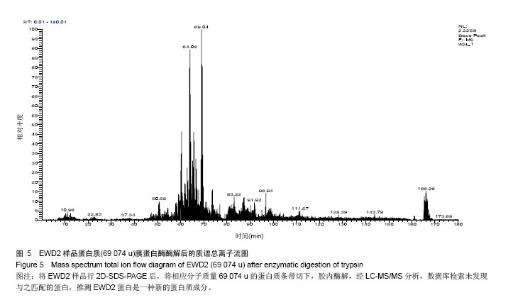
| [1] |
Shen Jinbo, Zhang Lin.
Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195.
|
| [2] |
Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong.
Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200.
|
| [3] |
Chai Le, Lü Jianlan, Hu Jintao, Hu Huahui, Xu Qingjun, Yu Jinwei, Quan Renfu.
Signal pathway variation after induction of inflammatory response in rats with acute spinal cord injury
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1218-1223.
|
| [4] |
Tan Jingyu, Liu Haiwen.
Genome-wide identification, classification and phylogenetic analysis of Fasciclin gene family for osteoblast specific factor 2
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1243-1248.
|
| [5] |
Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming.
Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055.
|
| [6] |
Wang Hanyue, Li Furong, Yang Xiaofei, Hu Chaofeng.
Direct reprogramming hepatocytes into islet-like cells by efficiently targeting and activating the endogenous genes
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1056-1063.
|
| [7] |
Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian.
Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148.
|
| [8] |
Zheng Xiaolong, He Xiaoming, Gong Shuidi, Pang Fengxiang, Yang Fan, He Wei, Liu Shaojun, Wei Qiushi.
Bone turnover characteristics in patients with alcohol-induced osteonecrosis of the femoral head
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 657-661.
|
| [9] |
Liu Xin, Yan Feihua, Hong Kunhao.
Delaying cartilage degeneration by regulating the expression of aquaporins in rats with knee osteoarthritis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 668-673.
|
| [10] |
Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song.
MicroRNA-138-5p regulates chondrocyte proliferation and autophagy
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678.
|
| [11] |
Xie Yang, Zhang Shujiang, Liu Menglan, Luo Ying, Yang Yang, Li Zuoxiao.
Mechanism by which rapamycin protects spinal cord neurons in experimental autoimmune encephalomyelitis mice
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 695-700.
|
| [12] |
Xu Yinqin, Shi Hongmei, Wang Guangyi.
Effects of Tongbi prescription hot compress combined with acupuncture on mRNA expressions of apoptosis-related genes,Caspase-3 and Bcl-2, in degenerative intervertebral discs
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 713-718.
|
| [13] |
Cao Xuhan, Bai Zixing, Sun Chengyi, Yang Yanjun, Sun Weidong.
Mechanism of “Ruxiang-Moyao” herbal pair in the treatment of knee osteoarthritis based on network pharmacology
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 746-753.
|
| [14] |
Liu Bo, Chen Xianghe, Yang Kang, Yu Huilin, Lu Pengcheng.
Mechanism of DNA methylation in exercise intervention for osteoporosis
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 791-797.
|
| [15] |
Deng Zhenhan, Huang Yong, Xiao Lulu, Chen Yulin, Zhu Weimin, Lu Wei, Wang Daping.
Role and application of bone morphogenetic proteins in articular cartilage regeneration
[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 798-806.
|